Our values shine through our research
Supported by more than 80 years of expertise in the probiotics field, Lallemand Health Solutions (LHS) has always considered Research & Development as a fundamental value from the very beginning.
An integrated research platform
From in vivo and in vitro models to clinical studies
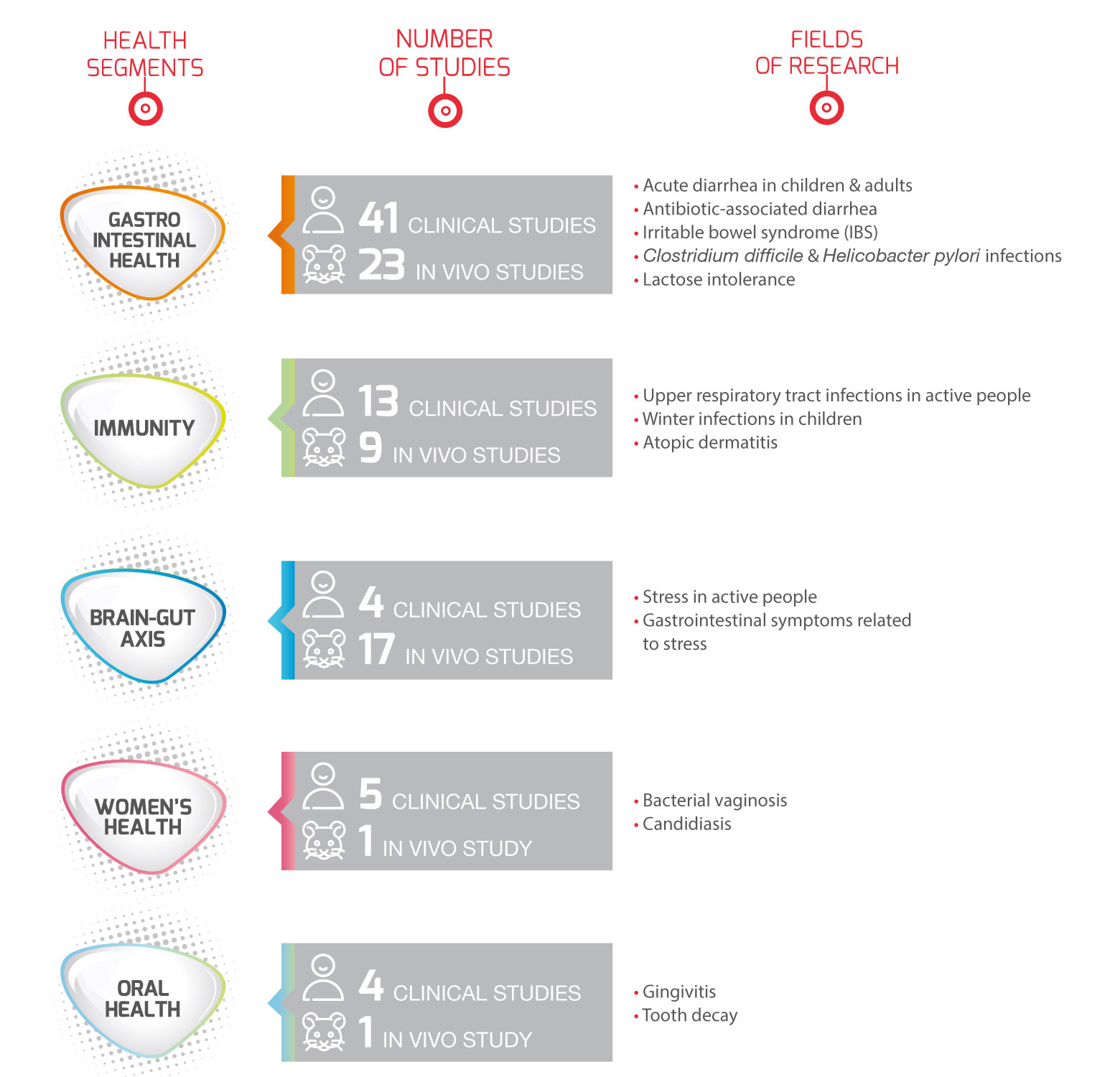
Our research at a glance
More than 260 papers published!
- 100+ in vitro
- 60+ in vivo
- 50+ clinical trials
Our areas of expertise

Probiotic bacteria & yeast production know-how
Clinical studies & safety
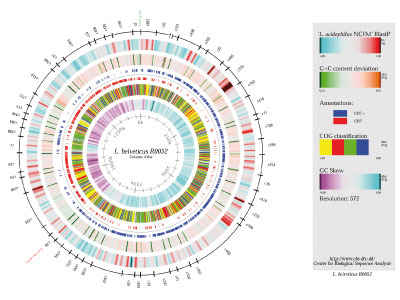
Genome sequencing (comparative & functional genomics)
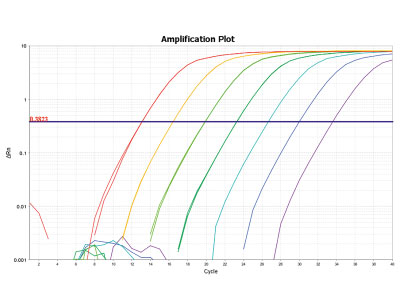
Quantification of probiotics in complex matrices (qPCR)
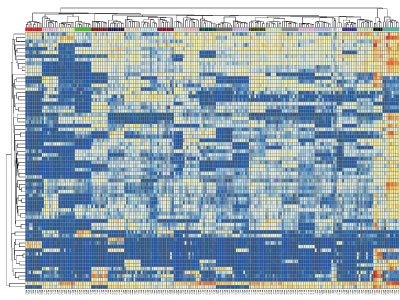
Microbiota analysis
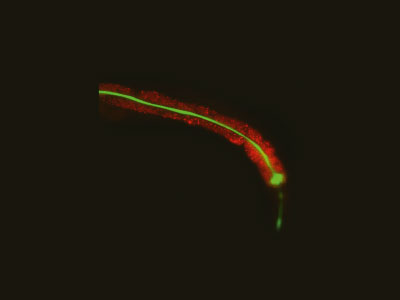
Animal screening model (Caenorhabditis elegans)
Host-microbe interactions
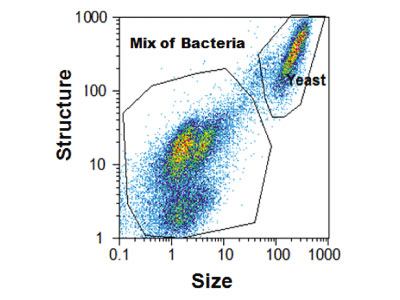
Quantification of probiotics in finished products (flow cytometry)
